GermanNetPaeT
German Network for Paediatric Trials
The GermanNetPaeT was founded in 2018 as part of the Innovative Medicines Initiative 2 (IMI 2) conect4children (c4c).
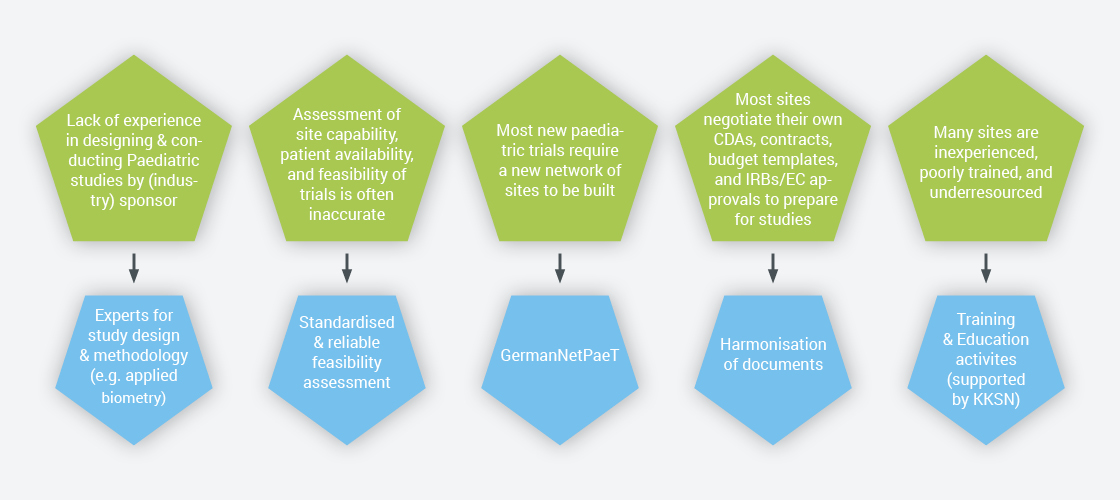
Aim of the GermanNetPaeT is to improve pharmacotherapy in paediatric patients regarding safety and efficacy by making clinical trials in children and adolescents more effective. Ultimate goal is to provide highly potent medicines for children and adolescents. Major prerequisite is an improved liaison between pharmaceutical industry and paediatric clinical study centres comprising all issues to conduct clinical trials. Particular interest is given on Harmonisation of Documents & Procedures, Ethics & Regulations, Pharmacovigilance, Training & Education, Applied Biometry and Patient & Parent Involvement.
One key feature of the GermanNetPaeT is the Single Point of Contact (SPoC) located at the head office at the Dr. Margarete Fischer-Bosch Institute of Clinical Pharmacology, Stuttgart.
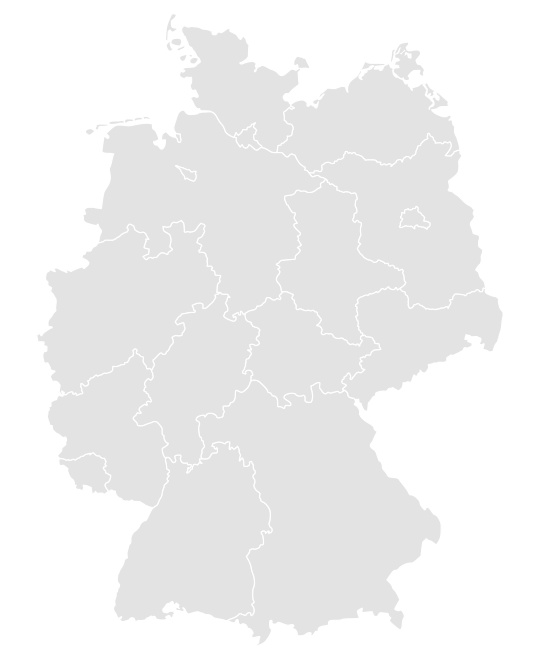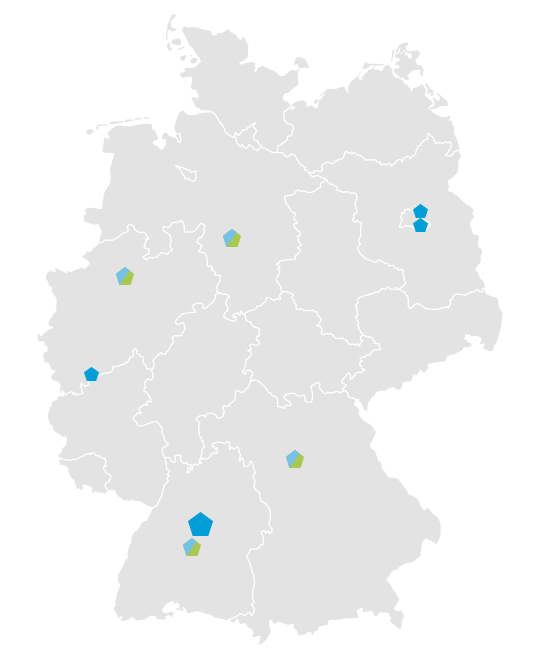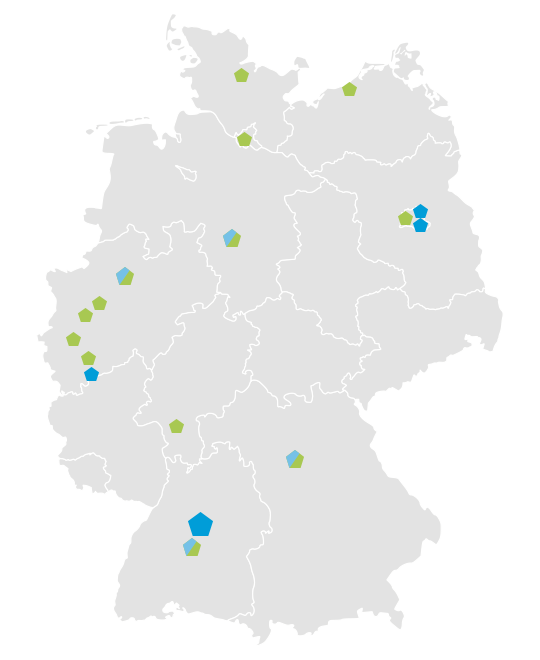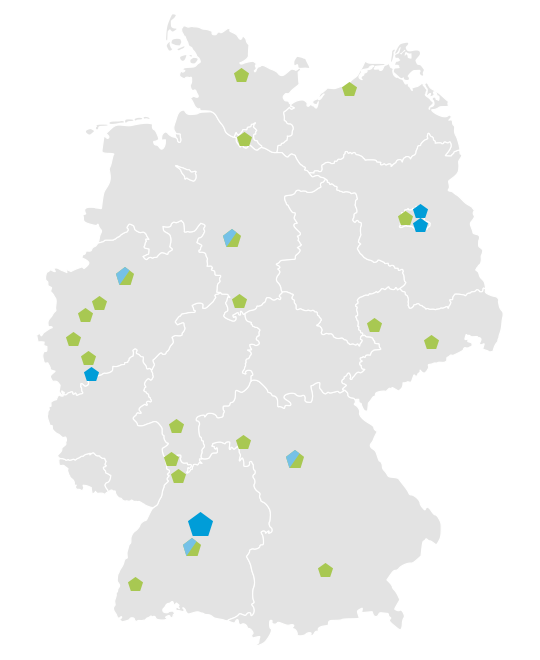
Currently more than 20 paediatric clinical study centres at hospitals for paediatrics and adolescent medicine and child and adolescent psychiatry are part of the GermanNetPaeT. The Network of Coordinating Centers for Clinical Studies (KKSN) strongly supports the GermanNetPaeT initiative. On behalf of the German Society of Pediatrics and Adolescent Medicine (DGKJ) all expert associations and working groups represented by the convent for collaboration of experts are affiliated. This guarantees the integration of experts for all paediatric subspecialities.
Patient and parent representatives are also actively involved.
Overcoming challenges when conducting paediatric trials